How To Draw The Lewis Structure
4.3: Drawing Lewis Structures
- Page ID
- 165659
- To draw Lewis structures.
- To recognize molecules that are probable to have multiple covalent bonds.
Cartoon LEWIS STRUCTURES
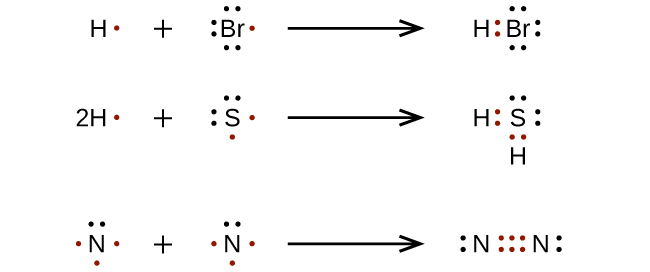
For very simple molecules and molecular ions, we tin write the Lewis structures by merely pairing up the unpaired electrons on the elective atoms. See these examples:
For more complicated molecules and molecular ions, it is helpful to follow the step-by-stride procedure outlined here:
- Determine the full number of valence (outer shell) electrons among all the atoms. For cations, decrease one electron for each positive charge. For anions, add together 1 electron for each negative accuse.
- Draw a skeleton structure of the molecule or ion, arranging the atoms effectually a central atom. (Generally, the to the lowest degree electronegative chemical element should exist placed in the center.) Connect each atom to the central atom with a single bail (i electron pair).
- Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen), completing an octet around each atom.
- Place all remaining electrons on the key atom.
- Rearrange the electrons of the outer atoms to brand multiple bonds with the central atom in club to obtain octets wherever possible.
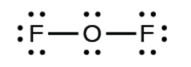
Let united states of america determine the Lewis structures of OF2 and HCN as examples in following this process:
one. Make up one's mind the full number of valence (outer shell) electrons in the molecule or ion. For a molecule, we add the number of valence electrons (apply the main grouping number) on each atom in the molecule. This is the total number of electrons that must be used in the Lewis structure.
O + 2 (F) = OF2
6e- + (ii x 7e-) = 20e-
H + C + Northward = HCN
1e-+ 4e-+ 5e-= 10e-
2. Describe a skeleton structure of the molecule or ion, arranging the atoms effectually a central atom and connecting each atom to the primal cantlet with a single (i electron pair) bond. Note that H and F can only form one bond, and are always on the periphery rather than the fundamental atom.
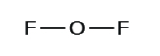
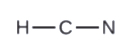
3. Distribute the remaining electrons equally lone pairs on the terminal atoms (except hydrogen) to complete their valence shells with an octet of electrons.
- In OFii, half-dozen electrons are placed on each F.
- In HCN, six electrons placed on Northward
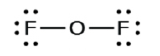
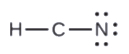
4. Identify all remaining electrons on the key cantlet.
- In OF2, four electrons are placed on O.
- In HCN: no electrons remain (the total valence of 10e-is reached) so nothing changes.
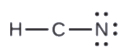
5. Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible.
- In OF2, each atom has an octet as drawn, and so null changes.
- In HCN, grade 2 more C–Northward bonds

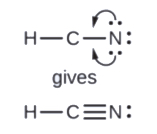
Finally, check to see if the full number of valence electrons are nowadays in the Lewis structure. Then, inspect if the H atom has two electrons surrounding information technology and if each of the principal group atoms is surrounded by viii electrons.
MULTIPLE BONDS
In many molecules, the octet rule would not be satisfied if each pair of bonded atoms shares only two electrons. Review HCN in Step five above. Another example is carbon dioxide (COii). CO2 has a total valence of 4e- + (two x 6e-) = 16e-. Following steps one to four, we describe the post-obit:
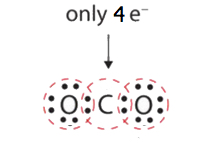
This does not requite the carbon atom a complete octet; only 4 electrons are in its valence shell. This arrangement of shared electrons is far from satisfactory.
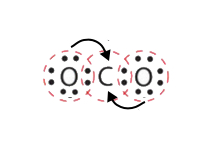
In this instance, more than one pair of electrons must be shared between two atoms for both atoms to have an octet. A second electron pair from each oxygen cantlet must be shared with the cardinal carbon atom shown by the arrows above. A lone pair from each O must be converted into a bonding pair of electrons.
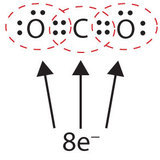
In this system, the carbon atom shares four electrons (two pairs) with the oxygen atom on the left and four electrons with the oxygen atom on the right. There are at present eight electrons around each atom. Two pairs of electrons shared between two atoms make a double bond between the atoms, which is represented by a double nuance:
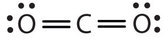
Some molecules comprise triple bonds (similar HCN, shown above). Triple bonds are covalent bonds in which three pairs of electrons are shared by two atoms. Another compound that has a triple bond is acetylene (C2H2), whose Lewis diagram is as follows:
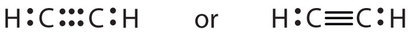
Draw the Lewis diagram for each molecule.
- \(\ce{N2}\)
- \(\ce{CH2O}\) (The carbon atom is the central cantlet.) Ane application of CHtwoO, likewise called formaldehyde, is the preservation of biological specimens. Aqueous solutions of CHtwoO are called formalin and have a sharp, characteristic (pungent) odor.
Solution
- The total number of electrons is two ten 5 = 10 electrons. The bond betwixt the two nitrogen atoms is a triple bond. The Lewis diagram for N2is as follows:
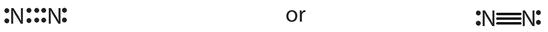
- The full number of electrons is 4 x ii(one) + half-dozen = 12 electrons. In CH2O, the central atom is surrounded by two dissimilar types of atoms. The Lewis diagram that fills each cantlet's valence electron shell is as follows:

Draw the Lewis diagram for each molecule.
- \(\ce{O2}\)
- \(\ce{C2H4}\)
- Answer a:
-
 or
or 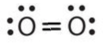
- Reply b:
-
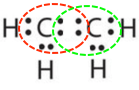 or
or 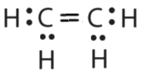 or
or 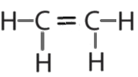
Which is the correct Lewis structure for N2H2?
A. 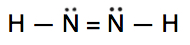
B. 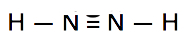
C. 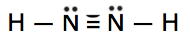
Solution
Lewis structure A is the correct answer. It has a total of (2 x 5e-) + (ii x 1e-) = 12e-. Each of the N atoms satisfy the octet requirement and the H atoms follow the duet rule.
Structure B is electron scarce. It has just 10e- instead of 12.
Structure C has fourteen (2 actress) electrons. The North atoms practise not satisfy the octet.
Exercise \(\PageIndex{2}\)
Which is the correct Lewis construction for NOCl?
A. 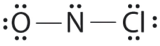
B. 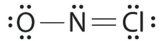
C. 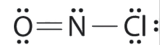
- Answer
-
Structure A violates the octet rule; N is surrounded by only 6e-.
Structure B violates the octet rule; Cl has 10e- around it. Furthermore, there are a total of 20e- instead of 18e-.
Structure C is the right structure. It has a total of 6e- + 5e- + 7e- = 18e-. Each atom is surrounded by viii electrons (octet rule).
Key Takeaways
- A Lewis structure shows the bonding and nonbonding electrons effectually individual atoms in a molecule.
- Some molecules must take multiple covalent bonds between atoms to satisfy the octet rule.
- A double bond contains four electrons and a triple bond contains six electrons.
Exercises
-
What is ane inkling that a molecule has a multiple bond?
2. Draw the Lewis diagram for each of the post-obit.
a. H2O
b. NH3
c. C2Hsix
d. CCl4
3. Each molecule contains double bonds. Depict the Lewis diagram for each. The first element is the central atom.
- CStwo
- C2F4
- COCl2
4. Each molecule contains multiple bonds. Draw the Lewis diagram for each. Assume that the beginning element is the central atom, unless otherwise noted.
- Nii
- CO
- HCN (The carbon atom is the central atom.)
- POCl (The phosphorus cantlet is the central atom.)
v. Explain why hydrogen atoms do not form double bonds.
6. Why is information technology incorrect to draw a double bond in the Lewis diagram for MgO?
Answers
- If single bonds betwixt all atoms do not give all atoms (except hydrogen) an octet, multiple covalent bonds may be present.
- a.
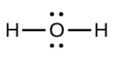
b.
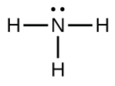
c.
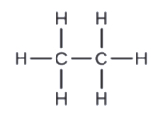
d.
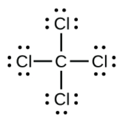
iv. a. 
b. 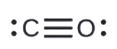
c. 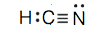
d.
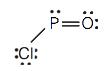
5. Hydrogen can accept only ane more electron; multiple bonds require more one electron pair to be shared.
6. MgO is an ionic compound (Mg transfers 2 electrons to O). The electrons are not shared hence it'southward incorrect to draw a double bail.
This is the Lewis dot construction of MgO.
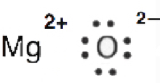
Source: https://chem.libretexts.org/Courses/Windward_Community_College/BIOC_141:_Fundamentals_of_Biochemistry_%28Colmenares_and_Ashburn%29/04:_Covalent_Bonding_and_Simple_Molecular_Compounds/4.3:_Drawing_Lewis_Structures
Posted by: cattplithenewark.blogspot.com


0 Response to "How To Draw The Lewis Structure"
Post a Comment